Why measure absorbance. Intensity is obtained using a spectrophotometer.
2 2 Ultraviolet And Visible Spectrophotometry ตำรายาของประเทศไทย กรมว ทยาศาสตร การแพทย
1
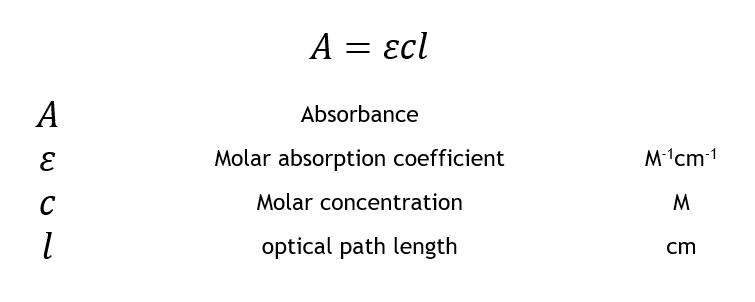
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
Y ax 2 bx c where solving for x determines the protein concentration of the sample.
Why is specific absorbance calculated. SPECTRA OF PARACETAMOL PCM 5. Machining Calculators should be used to calculate what speeds you should be machining your parts at by taking the material tool and tool material into account. Absorbance A The rate of decrease in the intensity of light with the thickness of the material the light is directly proportional to the intensity of the incident.
Some of the most common tools used for performance testing are listed here. This allows us to use a specific wavelength of light to detect the presence of. What are the top tools for performance testing.
When the molar coefficient and path length are constant absorbance is proportional to the concentration. Transmittance T 10. The intensity of the absorption varies as a function of frequency and this variation is the absorption spectrum.
Absorbance is the ratio of the negative logarithm of light intensity transmitted from a sample. If the data is collected using a reflection technique ATR reflection. It is given by the equation A log 10 I o I.
Molecules with an absorbance fluorescence excitation andor fluorescence emission spectrum sensitive to pH may be convenient probes for measuring pH i. In biology and chemistry the principle of absorbance is used to quantify absorbing molecules in solution. Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation as a function of frequency or wavelength due to its interaction with a sampleThe sample absorbs energy ie photons from the radiating field.
Because protein is readily degraded in the environment it is unknown whether protein-like substances in environments are a group of intact proteins or a mixture of peptides and even amino acids. For an absorbing substance and a specific wavelength the extinction coefficient ε is a constant specific usually the absorbance maximum of. The absorbance of a solution will change based on the wavelength that is passed through the solution.
The absorbance measurement is governed by Beers Law. Why you do not get complete sequence data for every protein. Where A is absorbance ε is the molar extinction coefficient b is the path length and c is the analyte concentration.
Absorbance value to a standard curve. Many biomolecules are absorbing at specific wavelengths themselves. The number of consecutive pi bonds.
Absorbance can also be calculated using the ratio between the intensity of a reference sample and the unknown sample. By also measuring the absorbance at specific wavelength the impurities can be detected. Why to consider Performance Testing.
In this work the widely adopted model protein bovine serum albumin BSA was compared with tryptones an assortment of peptides to evaluate their similarity with the proteins extracted from. The absorbance can be measured directly using the spectrophotometer or it can be calculated from the measurements of both the transmittance and reflectance by knowing the film thickness and the. Percent Transmittance Another key metric is absorbance that is defined as the amount of light absorbed.
Additional peaks can be observed due to impurities in the sample and it can be compared with that of standard raw material. IR absorbance spectroscopy obeys the BeerLambert law whereby band absorbance is linearly related to concentration of the molecule in solution. This is usually calculated as the negative of transmittance and is given by.
However absorbance assays are often an exception and a switch from a 3ml cuvette to 1ml micro-cuvette for example will not change the absorbance reading if the width path length of the cuvette is still 1 cm ie. Which enables the concentration of solution to be calculated by measuring its absorbance. Absorbance A C x L x Ɛ Concentration C AL x Ɛ The Lambert-Beer law describes the dependence of the absorbance on the concentration of the sample C the optical path length L as well as the dependence on a sample-specific extinction coefficient Ɛ which pertains to a specific substance at a.
If the data is collected in a transmission mode the y-axis should be in units of Transmittance T or absorbance abs. Must be adjusted to 100 transmittance 0 absorbance. Seeing enough peptides to show 70 of the sequence of a protein 70 coverageis a very successful protein analysis.
A εbc. If a solution containing a given dye is found to transmit 10 of the light when placed in a spectrophotometer its absorbance then would be calculated as follows. The absorbance was calculated as 100 transmittance.
Nucleic acids and proteins absorb UV light chlorophyll absorbs light of blue and orangered and hemoglobin absorbs yellow-green light. Absorption peak at a specific wavelength. Absorbance is calculated from the negative decadic logarithm of transmission.
The transmittance was recorded with spectrometer operating software. E hʋ equation 1 where E is the energy in joules h is Plancks constant 6626 x 10-34 Js and ʋ. 1 Why is a pencil used to mark the chromatogram and not a ballpoint or ink pen.
Performance testing is a non-functional type of testing and involves the process by which software or an application is tested to know its current system performance. Correctly setting up your tooling and material allows you to maximise machining quality as well as product quality. The light still passes through the same length of liquid.
In our last post we showed that molecules with C-C pi π bonds absorb light in the UV-visible region which promotes electrons from bonding π orbitals to anti bonding π orbitals. Percent transmittance of various dilutions of the bacterial culture is then measured and the values converted to optical density based on the formula. Absorbance OD 2 - log Transmittance.
Structure elucidation of organic compounds. One Hz is one cycle per second. In a project by the Cell Migration Consortium to analyze a number of protein involved in cell migration 80 coverage of a protein is considered sufficient.
This allows the estimation of the association constant and thereby ΔG by measuring the decrease in the intensity of the free AH band on formation of an H-bond along with knowledge of the total amounts of each substance present. The impure sample has lower specific activity because some of the mass is not actually enzyme. For instance in a standard cuvette the path length is 1 cm.
After 4 weeks of treatment the leaf transmittance at each LED treatment was obtained by scanning the light spectrum from 300 to 800 nm at an interval of 1 cm below a fully expanded leaf. Plotting a graph with the absorbance value as the dependent variable Y-axis and concentration as the independent variable X-axis results in an equation formatted as follows. If one exposes a solution of a pH-sensitive dye to light then dye molecules may absorb some of the light as electrons make the transition to a higher-energy state.
2 Explain why the cation samples are repeatedly spotted and dried on the chromatogram. This is done using a sample of uninoculated medium. The energy required for the transition depends mostly on the extent of conjugation ie.
The energy of light is calculated from frequency using equation 1. A Quick Review Of What Weve Learned So Far About UV-Vis. If the specific activity of 100 pure enzyme is known then an impure sample will have a lower specific activity allowing purity to be calculated and then getting a clear result.

Absorbance Measurements Bmg Labtech

Transmittance To Absorbance Table
Chem 125 Experiment Ii

Pdf Determination Of Specific Absorbance A For Zaleplon Sonata By Spectrophotometry Semantic Scholar

How To Calculate Molar Absorptivity 8 Steps With Pictures

What Is The Most Accepted Formula For Enzyme Activity Calculation
1
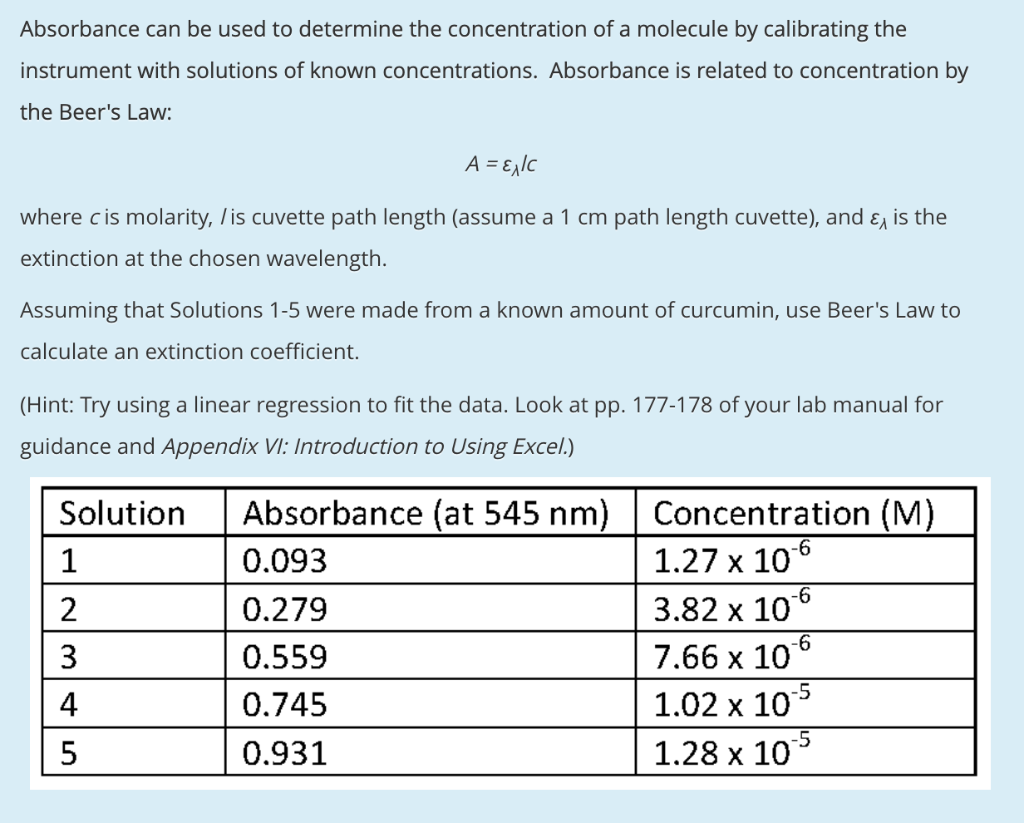
Solved Absorbance Can Be Used To Determine The Concentration Chegg Com
